COATS®-handskar visade en fyrfaldig ökning i hudens fukthalt jämfört med icke-belagda nitrilhandskar, med en anmärkningsvärd förbättring på 106,1% från baslinjen. COATS®-handskar visade en minskning av vattenavdunstning med 11,9% jämfört med en ökning av vattenavdunstning på 6,1% som observerades hos icke-belagda nitrilhandskar.
Teknologi
Vad är COATS®
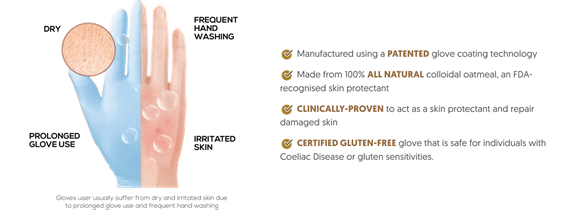
COATS® står för Colloidal Oatmeal System. Det är en patenterad handske-beläggningsteknik som innehåller ett kliniskt bevisat hudskyddsmedel.
Bortom skydd
Havremjöl har använts i århundraden som ett lugnande medel för att lindra klåda och irritation i samband med olika torra hudåkommor. I juni 2003 fick finfördelat havremjöl FDA-godkännande att användas som hudskyddsmedel. Genom att inse de unika fördelarna med finfördelat havremjöl kunde INNOVATIONSFÖRETAGET kombinera denna kunskap med sin innovativa handsktillverkningsteknologi för att erbjuda COATS® – den revolutionerande handsken som går bortom skydd.

WHY COLLOIDAL OATMEAL IS GREAT FOR SKIN?
GREAT PROTECTION FOR SENSITIVE SKIN
Colloidal oatmeal has been used for decades to soothe and ameliorate atopic dermatitis and other pruritic and/or xerotic dermatoses. The Centers for Disease Control and Prevention (CDC) also suggests that colloidal oatmeal baths could help relieve itching in cases of shingles (Herpes Zoster).3 Colloidal oatmeal’s properties are truly beneficial for glove users, in particular, the healthcare workers as according to a study in the British Journal of Dermatology published in July 2017, they are more likely to get hand eczema than the general population.
SOOTHING PROPERTIES
Colloidal oatmeal is the key to soothing sensitive skin, especially effective towards itches and irritations. Colloidal oatmeal binds to your skin and locks in moisture, giving your skin a chance to rehydrate. It also helps balance your skin’s natural pH levels.
ANTI-INFLAMMATORY PROPERTIES
In June 2003, colloidal oatmeal has gained the U.S. Food and Drug Administration (FDA) approval to be used as a skin protectant. Colloidal oatmeal has a long history of beneficial use in dermatology. Oatmeal possesses antioxidant and anti-inflammatory properties and its administration is effective on a variety of dermatologic inflammatory diseases.
MOISTURISING PROPERTIES
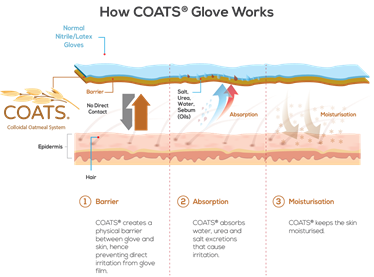
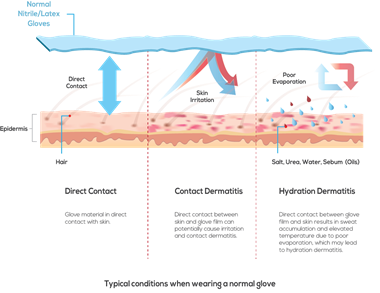
Hand washing is one of the most effective ways to prevent the spread of germs. However, frequent hand washing removes protective lipids from the skin barrier and as a result, the skin becomes less pliable and more prone to cracks and fissures. The high concentration of starches and beta-glucan in oats are factors leading to the protective and water-holding functions of oat.4 Colloidal oatmeal creates a film on top of the skin's surface, which enable reduction of Trans Epidermal Water Loss (TEWL) while preventing irritants from penetrating the skin.
BARRIER-REPAIR PROPERTIES
The finely grounded texture of Colloidal Oatmeal lets the molecules form a thin skin-like protective layer over the skin barrier that keeps moisture in and irritants out. Extracts of colloidal oatmeal were found to induce the expression of genes related to epidermal differentiation, tight junctions and lipid regulation in skin, and provide pH-buffering capacity. Colloidal oatmeal boosted the expression of multiple target genes related to skin barrier in an in vitro model of atopic dermatitis.
FEATURES
MARKET SEGMENT : Healthcare
TYPE : Examination
CUFF LENGHT : Standard
STERILE : NO
COLOR : Dawn blue, Lemon Green , White
AQL : 1.5
REGULATORY COMPLIANCE : Nitrile Powder Free : FDA 510(k), ROHS Directive 2002/95/EC, EU 2016/425, REACH, EU 10/2011, EC 1935/2004, MDR 2017/ 745
Latex Powder Free: FDA 510(k), REACH, ROHS Directive 2002/95/EC, EU 10/2011, EC 1935/2004, MDR 2017/ 745
STANDARDS : Nitrile Powder Free: ASTM D6319, ASTM D6124, ASTM D5151, ASTM F1671, ASTM D6978, EN 455 part 1, 2 & 3, EN 1186, EN 13130, CEN/TS 14234, EN 420, EN ISO 374-1, EN 16523-1, EN ISO part 2, 4 & 5, ISO 10993 part 5, 10, 11 & 23
Latex Powder Free: ASTM D5151, ASTM D6124, ASTM D3578, ASTM D5712, EN ISO 374-1 (Type B), EN 16523-1, EN ISO part 2, 4 & 5, EN 420, EN 455 part 1, 2, 3 & 4, EN 1186, EN 13130, CEN/TS 14234, ISO 10993 part 5, 10, 11 & 23
CLASSIFICATION : Nitrile Powder Free: Class I (FDA), Class I (MDR 2017/745), Category 3 (BfR XXI), Category III (PPER 2016/425)
Latex Powder Free : Class I (FDA), Class I (MDR 2017/745), Category 3 (BfR XXI), Category III (PPER 2016/425)
